Abstract
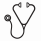
Background : Lenalidomide (Len) and dexamethasone (Dex) combination (Ld) therapy is now the standard treatment option for multiple myeloma (MM). MM mainly affects elderly individuals, and about 40% of MM patients are diagnosed over 75 years of age. Many elderly patients are vulnerable and therefore they are susceptible to adverse events (AEs). Therefore optimal dose intensity is needed when we treat elderly patients. Renal impairment (RI) is the most common complication of MM and 50% of MM patients develop RI during the clinical course. Len is excreted through urine and dose adjustment is required according to creatinine clearance (CCr) to prevent overexposure of Len. However despite the initial dose adjustment of Len according to CCr, unexpected adverse events (AEs) can occur. AEs are consistent with Len overexposure; however, optimal plasma concentration has not been established yet. Furthermore Len is known as an immunomodulatory drug and which can enhance the NK cell cytotoxicity as represented by the combination of elotuzumab.
Purpose : We conducted a phase II trial of Ld therapy in elderly patients with newly diagnosed MM to evaluate the correlation between its efficacy, safety and Len pharmacokinetics and the immunomodulatory effect of Len against NK cells.
Methods : Forty Japanese patients received oral Len on days 1-21 of a 28-day cycle and Dex weekly. The initial dose of Len was adjusted according to CCr [25 mg/day for normal renal function, 10 mg/day for mild RI (CCr 30- 60 mL/min) and 15 mg every other day for severe RI (CCr < 30 mL/min)]. The initial dose of Dex was adjusted based on patient's age [40 mg for ≤75 years and 20 mg for >75 years]. Some dose modification of Len and Dex was permitted by physician's choice if judged necessary considering the vulnerability. Whole-blood samples were collected immediately before oral administration (C0h) and 2, 4(C4h), 12, and 24 h thereafter and analyzed using liquid chromatography-tandem mass spectrometry. We predicted the AUC0−24 of Len using a previously developed formula, AUC0−24 = 37.1 × C0h + 6.4 × C4h - 32.1 × CCr + 3265.6; r2 = 0.842 (Ther Drug Monit 2014). Peripheral blood mononuclear cells (PBMCs) were used to evaluate the immunomodulatory effect of Len against NK cells. This study protocol was approved by the Ethics Committee of Akita University Hospital, and all recipients gave written informed consent.
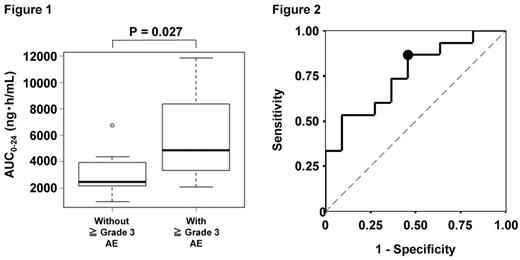
Results : Median age was 75.5 years and 21 patients showed RI necessary to adjust the Len dosage. The median initial dosage of Len and Dex was 12.5 mg and 20 mg, respectively. Overall response rate was 68.6 % and 2-year overall survival was 88.5%. The predicted AUC0-24 showed the highest correlation with the measured AUC0-24 ( r = 0.956; P < 0.001). There was no correlation between response and Len pharmacokinetics. Grade 3 to 4 adverse events (AEs) were observed in 57.5% of patients and AUC0-24 was significantly higher in patients with grade 3 to 4 AEs than in those without grade 3 to 4 AEs (median 4852.0 ng · hr/mL vs. 2464.9 ng · hr/mL, P=0.027) (Figure 1). We identified a cut-off value of AUC0-24 for predicting grade 3 to 4 AEs using a receiver-operating characteristic curve and the value was 2613.5 ng · hr/mL (area under ROC curve of 0.758, p = 0.027) (Figure 2). Multivariate logistic analysis confirmed the significance of this cut-off value as a predictor of grade 3 to 4 AEs (hazard ratio 7.20, P = 0.043). The patients who achieved more than partial response (PR) showed the increased frequency and number of CD25 positive NK cells. However, there were no correlations between NK cells and PK parameters.
Conclusions : These data suggest that Len overexposure could contribute to toxicity and the predicted cut-off AUC0-24 can be useful in preventing grade 3 to 4 AEs. Activated NK cells were increased in patients who achieved more than PR than in patients who did not achieve.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract